QuantaDose Press Releases
FDA’s “Cell Phones” Page Quietly Changed—and Now It Reads Like a Plain-Language Restatement of PL 90-602
In mid-January 2026, reporting indicated that the U.S. Department of Health and Human Services (HHS) directed a new study on electromagnetic radiation and health effects, and that the U.S. Food and Drug Administration (FDA) removed webpages containing “old conclusions” about cellphone radiation in order to identify knowledge gaps, including those tied to emerging technologies.
What replaced at least one of the most-cited FDA pages is not a new “all-clear” message or a new “hazard” declaration. Instead, the current FDA content is dominated by a description of the agency’s legal responsibilities—language that closely tracks the structure and intent of Public Law 90-602, the Radiation Control for Health and Safety Act of 1968.
What changed: from “conclusions” to “statutory responsibilities”
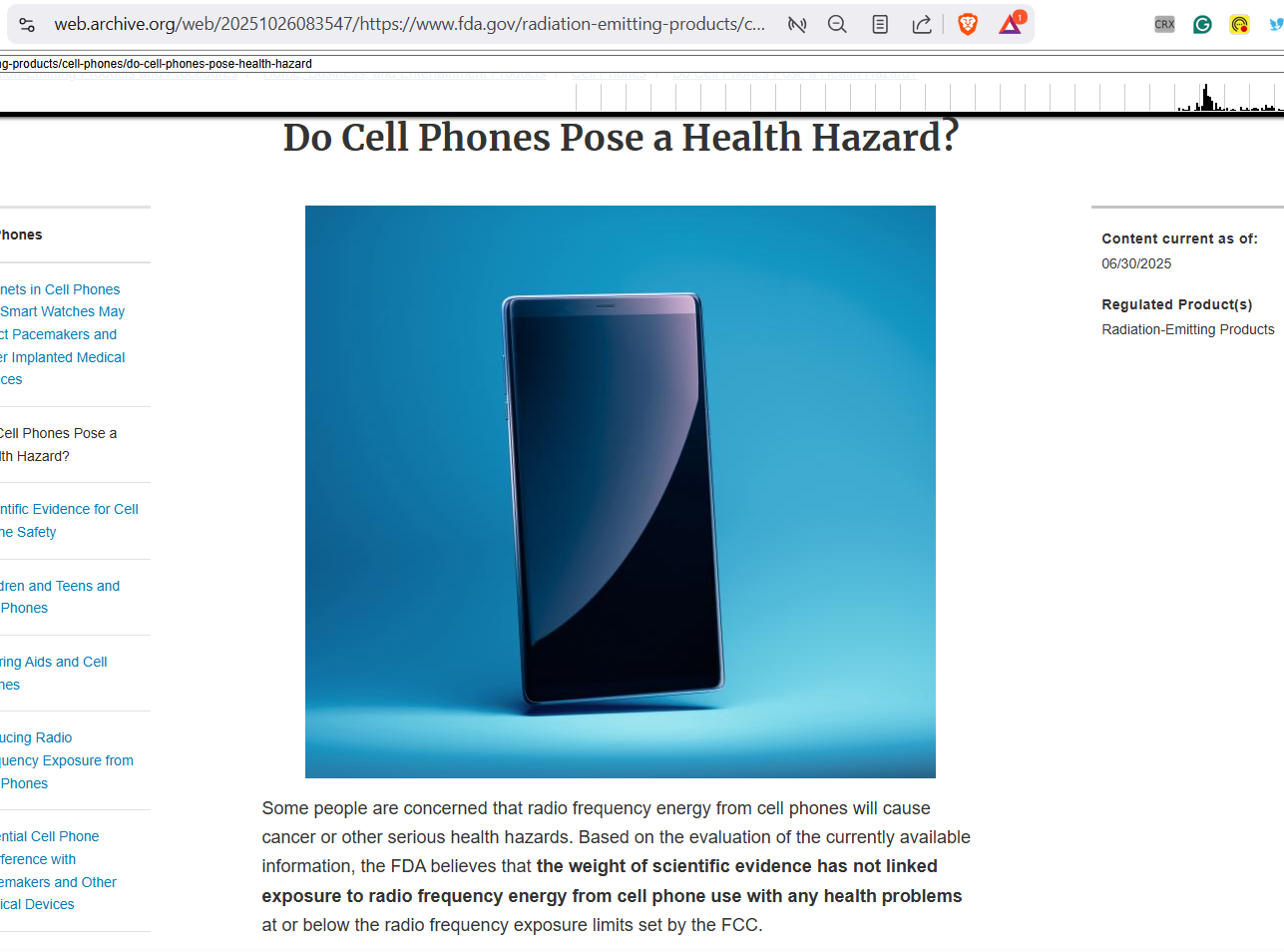
The FDA URL historically associated with “Do Cell Phones Pose a Health Hazard?” now resolves to the FDA’s main “Cell Phones” page. That page begins with a regulatory framing: the FDA shares responsibilities with the FCC, and “under the law” the FDA is responsible for, among other things, consulting with other agencies on testing and evaluation and “collecting, analyzing, and making available scientific information on the nature and extent of the hazards and control of electronic product radiation.”
The “Do Cell Phones Pose a Health Hazard?” concept still exists on the page, but only as a short sub-topic sentence rather than a long-form explainer.
Why the wording matters: it mirrors PL 90-602 in substance and structure
https://www.fda.gov/radiation-emitting-products/home-business-and-entertainment-products/cell-phones
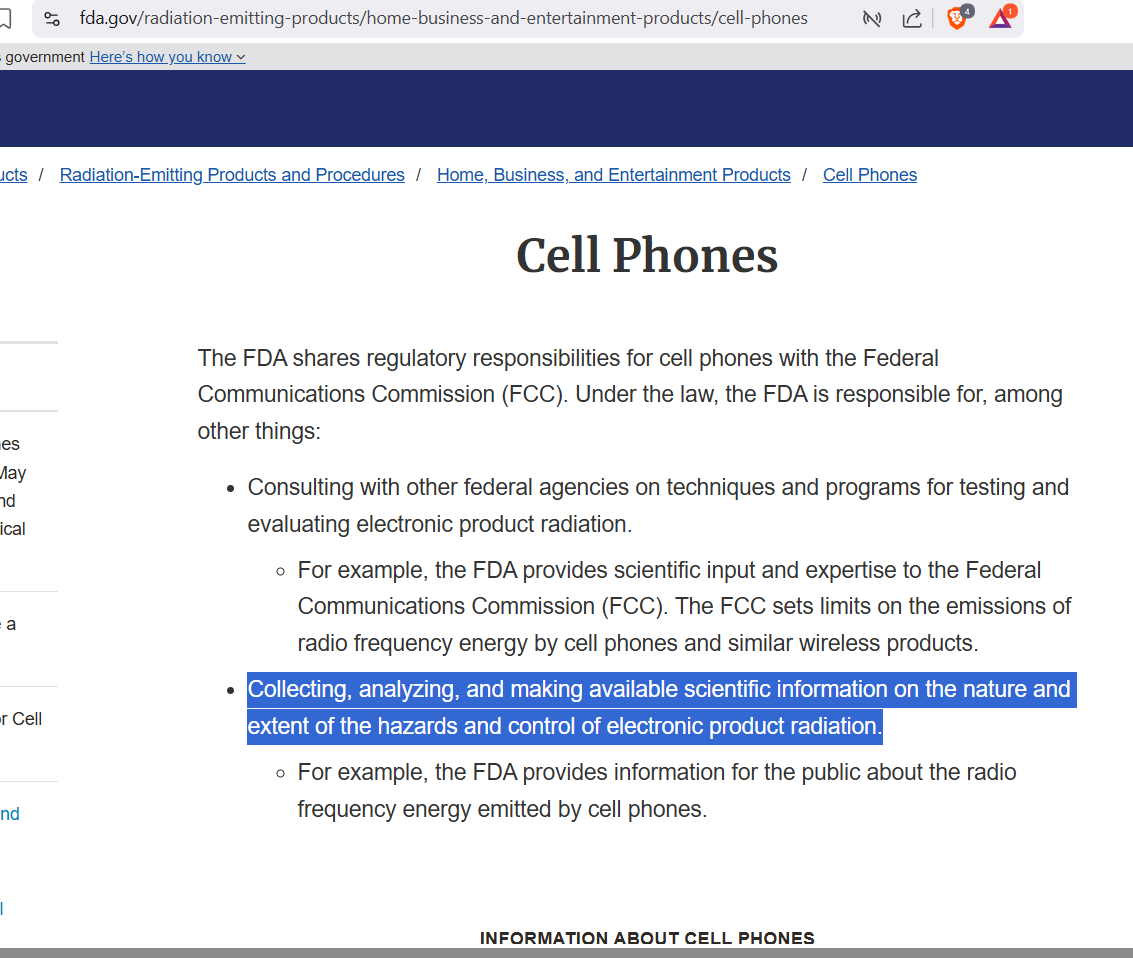
The key bullet on the current FDA page—“Collecting, analyzing, and making available scientific information on the nature and extent of the hazards and control of electronic product radiation”—is striking because it reads like a modern paraphrase of the research-and-publication mandate spelled out in PL 90-602.
In the statute, Congress directs an electronic product radiation control program and explicitly authorizes the Secretary to “collect and make available” the results of research and other information relating to “the nature and extent of the hazards and control of electronic product radiation.”
The law’s purpose clause also makes the program’s objective unambiguous: public health and safety “must be protected from the dangers of electronic product radiation,” and the program is intended to include both standards and research into the effects and control of radiation emissions.
In short: the FDA’s revised “Cell Phones” framing is not merely a web refresh. It re-centers the government’s role around the statutory mission—continuous evaluation, research support, and public-facing dissemination of information on hazards and control.
The “old conclusions” that were historically emphasized
The “reopening” narrative makes sense because the FDA’s earlier long-form messaging was widely circulated for years. It included strong, confidence-forward formulations such as the repeated line that the “weight of scientific evidence has not linked” cellphone use with health problems—language that appears in government records and third-party documentation quoting the FDA’s prior page text.
Recent reporting described the removal of webpages that had communicated those older conclusions, while also noting that some FDA and CDC webpages still contain versions of the same safety assurances.
Importantly, the current FDA “Cell Phones” page itself still includes condensed statements that the “weight of scientific evidence has not linked” RF exposure from cell phones to health problems and that evidence does not show danger to children and teens—yet the surrounding page architecture now foregrounds legal duties and agency functions rather than extended dismissal narratives.
Why RF Safe views this as a compliance milestone
RF Safe has argued for years that PL 90-602 is not optional rhetoric but a binding framework: a continuing federal obligation to evaluate hazards from electronic product radiation, to support research, and to keep the public informed as technologies evolve. The current FDA “Cell Phones” page now places that statutory framing at the top of the public-facing presentation.
RF Safe’s interpretation is that the January 2026 shift—HHS commissioning work to identify knowledge gaps and FDA removing “old conclusions” pages—moves federal messaging closer to the law’s intended posture: active, iterative oversight rather than a static, closed-case narrative.
That is a claim about intent and direction, not a definitive legal finding of past compliance or noncompliance. But as a public signal, the revised FDA framing clearly aligns more directly with the language Congress wrote into PL 90-602.
What to watch next
If this change is more than cosmetic, the next indicators will be operational rather than rhetorical:
-
Whether the HHS-directed study publishes a defined scope, leadership, and transparency rules (methods, conflicts, deliverables).
-
Whether FDA updates remain limited to brief “weight of evidence” lines, or whether they evolve into clearer consumer-facing explanations of uncertainties, limitations, and practical risk-management guidance.
-
Whether the statutory posture emphasized on the new page is followed by measurable follow-through: research activity, public reporting, and clearly stated knowledge gaps consistent with the “collect and make available” mandate.
Bottom line
The most telling detail is not merely that a page was removed. It is what replaced it.
The FDA’s current “Cell Phones” page now leads with a mission statement that reads like a plain-language restatement of PL 90-602: consult on evaluation, and collect and make available scientific information about hazards and control of electronic product radiation.
In the broader January 2026 context—HHS announcing a new study and describing the removal of “old conclusions” webpages—this shift signals a meaningful reframing: from “settled conclusions” toward statutory obligations and renewed inquiry.
